Gpi Pharma supplied a 33 m³ horizontal and a 5 m³ vertical stainless steel tank for purified water (WFI) for application in the cosmetics industry.
Our client specializes in high-end technical solutions for the pharmaceutical industry. The company designs and delivers machinery and systems for the packaging and processing of pharmaceuticals and cosmetics. For an expansion project at a cosmetics manufacturer, Gpi Pharma was commissioned to design, manufacture and deliver two new stainless steel WFI tanks.
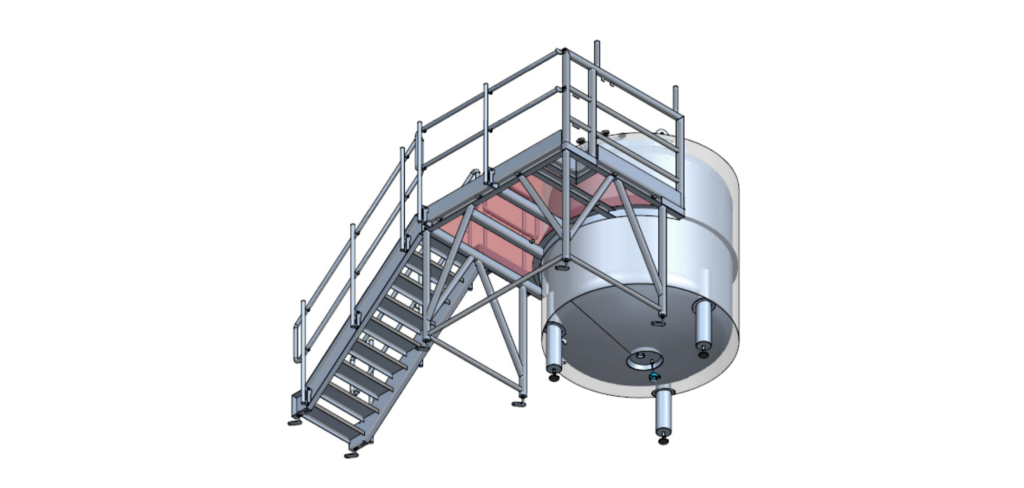
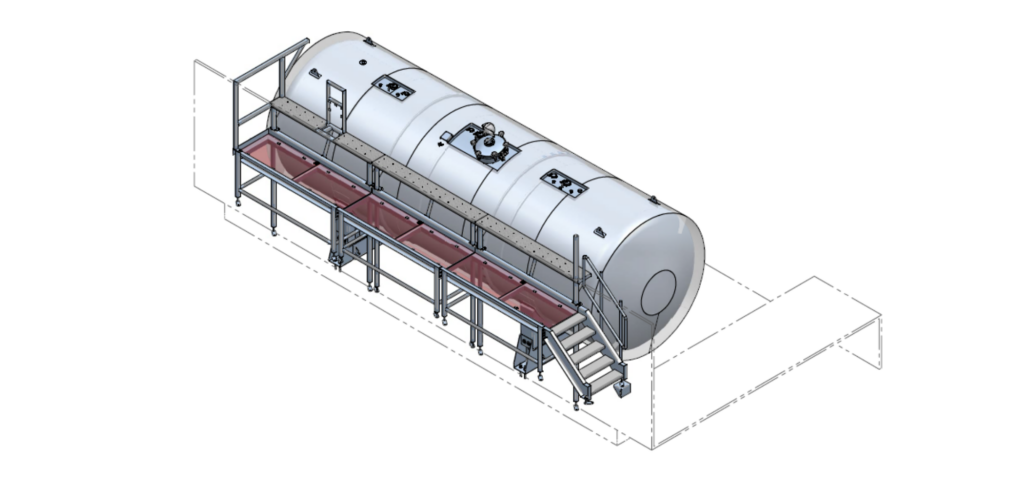
The request included a 33 m³ horizontal WFI tank, a 5 m³ vertical storage tank, and two platforms to ensure safe access and efficient maintenance. Gpi Pharma delivered this complete solution.
About the project
For this project, Gpi Pharma engineered, manufactured and delivered two hygienic stainless steel tanks including custom access platforms. The tanks are fabricated from high-grade 316L stainless steel and comply with the PED directive and the EN 13445 design standard. Two tailor-made platforms were supplied to ensure safe and efficient access.
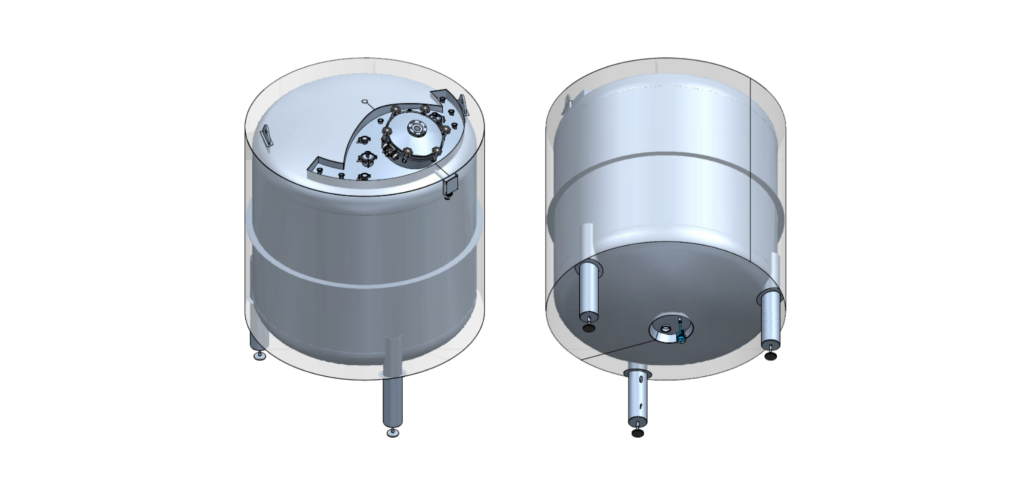
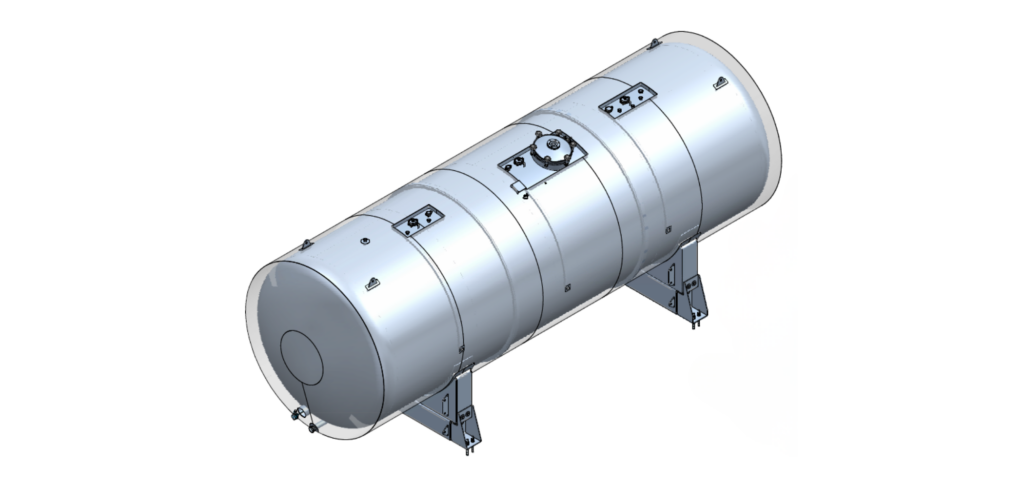
The horizontal WFI tank has a working volume of 33 m³, a diameter of 2.6 meters and a length of 6 meters, and is equipped with a high-pressure top and bottom. The vertical storage tank has a capacity of 5 m³, a diameter of 2 meters and a height of 1.5 meters. Both tanks are designed for the storage of WFI (Water for Injection), purified water used in the production of cosmetic and aesthetic products.





Production, inspection and transport
Following drawing approval, production commenced at Gpi’s manufacturing facility in Lopik. In accordance with the agreed specifications, several tests were performed to guarantee the quality and cleanability of the tanks.
A visual inspection and a surface crack test were carried out to detect any potential microcracks on the internal surfaces, which are critical for safety and durability. In addition, a riboflavin test was performed in combination with a drain test. Using RO water, it was demonstrated that all internal surfaces are fully cleanable and that the tanks completely drain after cleaning. This confirms compliance with pharmaceutical cleanability standards.
After final approval, the tanks and platforms were transported from Lopik to the client. During the engineering phase, careful consideration was given to transport logistics and installation within the end customer’s facility. The tanks had to be installed in a basement area, presenting a logistical challenge that was resolved through the use of split saddles, among other solutions. A proactive engineering approach ensured smooth on-site installation. The final installation was carried out by the client.





Documentation
Gpi Pharma managed the entire scope of supply, including all required technical documentation. The documentation requirements for this project were particularly stringent. In close collaboration with the client, the documentation package was developed to the required level of detail, enabling full compliance upon handover to the end user.
In addition to a complete welder’s log and standard 3.1 material certificates, FDA and USP Class VI certifications were provided. Additional certifications were supplied for the sealing materials, confirming that they are free from BPA and DEHP, contain no NRL/DNR, and comply with TSE, BSE and AOF (Animal Origin Free) regulations.
The result
The project resulted in a reliable and fully compliant WFI storage solution within a strictly regulated production environment. The new tanks and platforms integrate seamlessly into the existing installation and support the further expansion of the end user’s production capacity.
By addressing transport logistics, basement installation constraints and extensive documentation requirements from the early engineering phase onward, the project was executed efficiently and delivered without delays. The combination of technical precision, validated cleanability and comprehensive certification provides the end user with full assurance of compliance with all applicable quality and regulatory standards.










